Originally published at: https://www.pakwheels.com/blog/catalytic-converter/
Today we are going to shed some light on what catalytic converters are, how do they work, what are they made up of and why are they so important so without further ado let’s take a detailed look at catalytic converters.
What does a catalytic converter do?
Catalytic converters are designed to reduce harmful emissions. It is basically an emission control device that converts harmful gasses and pollutants in the exhaust gasses into less toxic gasses by a redox reaction (reduction and oxidation). Since 1970 governments around the work have been pushing automobile manufacturers to build cars with lower emissions. Today, almost all of the developed countries have regulations which prohibit manufacturers on building cars without catalytic converters.
Cars without catalytic converters release various harmful gasses like carbon monoxide, nitrogen oxide and hydrocarbons which cause smog and are dangerous for our health. Every modern car, bus, truck and even generators have some form of catalytic converter to reduce the amount of harmful gasses from releasing in our atmosphere.
There are two basic types of catalytic converters:
- a two-way converter
- a three-way converter
We will be discussing about three-way converter as two-way catalytic converts are a thing of the past now.
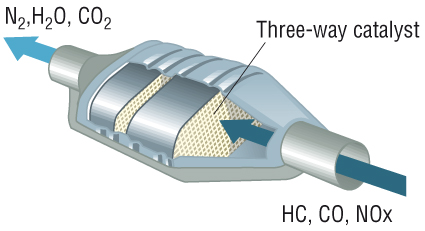
What is it made of?
The catalytic converter is basically a metal shell filled with ceramic and some catalyst. The core on the inside is ceramic in the shape of a honeycomb structure. The catalyst itself is a mixture of rare precious metals. Platinum is the most active catalyst and widely used, but less suitable as its expensive so palladium and rhodium are also used. Rhodium is used as a reduction catalyst and palladium is used as an oxidation catalyst while platinum is used for both reduction and oxidation. Some other catalysts that were previously used are iron, manganese, cerium, and nickel, but aren’t used anymore due to limitations.
How does it work?
So the petrol is made up of hydrocarbons. Inside an internal combustion engine if the efficiency is 100%, then all those hydrogen molecules combine with carbon and oxygen molecules to produce carbon dioxide and water. But the thing is, the combustion in an engine is not 100% perfect resulting in production of a harmful gas knows as carbon monoxide. Also, a possibility is unburnt fuel, meaning that hydrocarbons also exit from the exhaust into the atmosphere which are also dangerous.
The catalyst helps to convert those harmful gasses to less dangerous ones. The ceramic inside doesn’t react with anything until its hot (800 degrees Fahrenheit or more than 400 degrees Celsius) which is achieved by the hot exhaust gasses from the engine. Inside the catalytic converter you have two blocks: a reduction catalyst made of platinum and rhodium and an oxidation catalyst made of platinum and palladium. So, after combustion you have carbon dioxide, carbon monoxide, nitrogen oxide, oxygen, water and unburnt fuel all coming into the catalytic converter.
First stage
In the first step it goes through the reduction catalyst and its purpose is to eliminate nitrogen oxide. So, the nitrogen oxide that comes in has nitrogen and oxygen molecules and their bond isn’t that strong as compared to a bond between nitrogen and the catalyst so it will bond with the catalyst weakening its already made bond freeing those oxygen molecules which will pass on to the next catalyst as separate oxygen molecules or combine together. The nitrogen bond is not as strong with the catalyst as compared to a bond with another nitrogen molecule so further two nitrogen molecules will bond together, breaking the nitrogen and catalyst bond and pass away as nitrogen to the second catalyst. Now you have simple oxygen and nitrogen molecules which are completely harmless and the catalyst will keep performing its reactions with more incoming gasses.
2 CO + 2 NO → 2 CO2 + N2
2 H2 + 2 NO → 2 H2O + N2
Second stage
The second step is the oxidation catalyst and its purpose is to eliminate carbon monoxide. The catalyst tries to bond with oxygen and breaks the bond between oxygen molecules and creates separate oxygen molecules which have a stronger bond with carbon monoxide creating carbon dioxide which will freely exit the catalytic converter. Same is he case with all the unburnt fuel. It combines with oxygen and form water and carbon dioxide and this process is repeated with more incoming gasses.
CO + O2 → 2 CO2
Hydrocarbon + O2 → H2O + CO2
Now in the end you are just left with nitrogen, oxygen, carbon dioxide and water and the average efficiency of catalytic converters are 90% and that is how catalytic converters work.
The catalytic converter is an intergrtal part of any modern vehicle. Removing it not recommended at all. Most imported car owners know how important the catalytic converter is. Locally produced automobiles don't relay on catalytic converter that heavily as all these imported Japanese vehicles do. Our locally available fuel is on inferior quality in many ways. It can chock the catalytic honeycomb inside the converter body with all sorts of deposits, resulting in the overall decrease in the performance of the car as well as the fuel average. In case that happens, it is better to get it cleaned or replaced in extreme cases, rather than removing it altogether.